Meningitis Linked to Compounded Steroid Drugs
An outbreak of meningitis infections caused by contaminated epidural steroid injections is concerning doctors. Let’s look at the basis for the concern.
In this article
Contaminated steroid injections
The core issue revolves around the methods of compounding drugs. A less discussed concern is whether steroid injections for low back pain may produce more problems than solutions when used on a long-term basis.
According to the Centers for Disease Control, the contaminated drug has caused meningitis infections in at least 185 people and killed 14 people in twelve states in 2012. The CDC announced that the injections may also have unknowingly caused strokes, septic arthritis and spinal osteomyelitis among infected patients. Symptoms may include fevers, headaches, light sensitivity and stiff neck. The unknown factor is how long the infections will take to cause damage. For many the infection may have started with injections received as early as May 21st, 2012.
The CDC warned that infections of typically protected areas of the body, such as cerebral spinal fluid, joint fluids, lymph nodes, brain, pericardial fluid or pleural fluids should be suspected as possible meningitis infections caused by the contaminated steroid medications.
Compounded injections not approved
Three lots of compounded methylprednisolone acetate doses of 80 milligrams per milliliter were apparently contaminated by the Exserohilum fungus, and quite possibly also Aspergillus. The CDC found the Exserohilum fungus among 10 of the meningitis patients tested and Aspergillus fungus in one infection. The FDA now suspects that the contamination occurred at a single compounding pharmacy in Massachusetts.
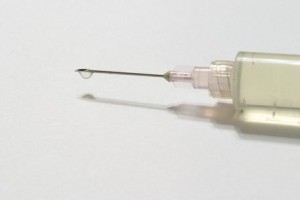
The epidural steroids are injected into the foramina – openings in the vertebra positioned around the spinal nerve where external nerves meet the spine. The idea is that the steroids may reduce the inflammation surrounding the region, theoretically the cause of nerve irritation and low back pain.
While the U.S. Federal Drug Administration has approved some epidural steroid injections, they have not approved the injection of compounded methylprednisolone acetate.
And there is certainly cause for concern about the use of methylprednisolone acetate. In a study published in January in the medical journal Anesthesiology, German researchers found that weekly injections with methylprednisolone acetate given to dogs for six weeks caused a significant increase in inflammation among those given the equivalent to human doses, and those given higher doses were found to have “large inflammatory masses.”
These findings caused the researchers to conclude that, “These results, showing dose-dependent intrathecal inflammatory reactions at MPA doses and injectate concentrations comparable to those used in humans, indicate that the continued use of this modality in humans is not recommended.”
Dangers of steroids
This is in addition to the dangers of long-term use of powerful steroids in general – especially those injected directly into the spine. Side effects that have been documented for methylprednisolone include a variety of muscle and bone weaknesses, including tendon ruptures, fractures, bone disintegration and others. They also include encephalitis and nerve damage, headaches, vertigo and convulsions.
Because methylprednisolone is a steroid that mimics cortisol produced by the adrenal glands, its long-term use either in injection form or oral form can cause various endocrine issues, including Cushing’s syndrome, growth problems, menstrual cycle issues, insulin issues and pituitary problems. Meanwhile, thinning of the skin can produce sweating problems, and increased eye pressure and glaucoma have been seen in some cases. Various digestive issues and nutrient loss have also been seen, and slow healing is often a result of long-term steroid use.
In addition to these risks, there is a risk that the injection will hit a spinal artery or another spinal innervation, causing an array of possible paralytic issues.
Another reason cited for the dangers of compounded methylprednisolone acetate is the compounding of polyethylene glycol within the injection dose. Polyethylene glycol has been shown to cause a wide range of negative effects, including hives, breathing issues, itching, swollen tongue and other types of allergic reactions.
Weak evidence for pain relief
Meanwhile, research has shown that injection of methylprednisolone acetate may only produce “modest” and short-term relief for back pain. In a study of 84 adults with low back pain, 75% of those receiving the steroid injections had some short-term pain relief, compared to 50% of those receiving saline with the same level of pain relief. The researchers concluded that, “Epidural steroid injections may provide modest short-term pain relief for some adults…”
The question this brings up is whether it is worth risking long-term nerve damage, brain damage and even death to receive a “modest” amount of short-term pain relief.
Meanwhile an array of natural pain and inflammation treatments have been safely used for thousands of years for joint and low back pain. There are also a number of herbs proven to reduce pain.
REFERENCES:
Rijsdijk M, van Wijck AJ, Kalkman CJ, Meulenhoff PC, Grafe MR, Steinauer J, Yaksh TL. Safety assessment and pharmacokinetics of intrathecal methylprednisolone acetate in dogs. Anesthesiology. 2012 Jan;116(1):170-81.
Candido KD, Knezevic I, Mukalel J, Knezevic NN. Enhancing the relative safety of ethylprednisolone administration by removing polyethylene glycol. Anesth Analg. 2011 Dec;113(6):1487-9.
Cohen SP, White RL, Kurihara C, Larkin TM, Chang A, Griffith SR, Gilligan C, Larkin R, Morlando B, Pasquina PF, Yaksh TL, Nguyen C. Epidural steroids, etanercept, or saline in subacute sciatica: a multicenter, randomized trial. Ann Intern Med. 2012 Apr 17;156(8):551-9.
Atanasković-Marković M, Gavrović-Jankulović M, Janković S, Blagojević G, Cirković-Velicković T, Milojević I, Simić D, Nestorović B. Immediate allergic reaction to methylprednisolone with tolerance of other corticosteroids. Srp Arh Celok Lek. 2012 Mar-Apr;140(3-4):233-5.